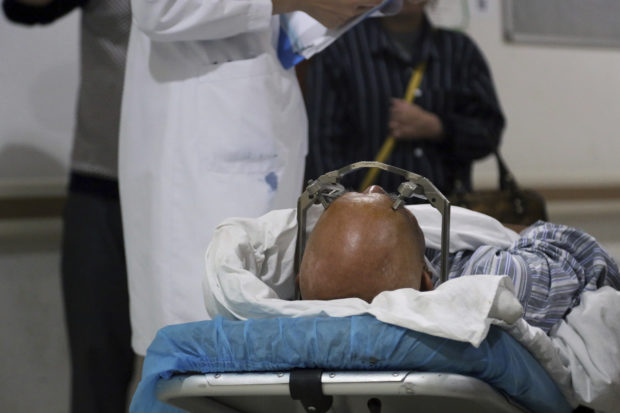
A stereotactic device presses into the head of a brain surgery patient at Ruijin Hospital’s functional neurosurgery center in Shanghai, China on Monday, Oct. 29, 2018. AP
SHANGHAI — The sound of doctors boring through his skull to feed electrodes deep into his brain made Yan tremble. “The drill was like bzzzzzzz,” he later recalled. “The moment of drilling is the most terrible.”
Yan is a methamphetamine addict. The hope is that technology will extinguish his addiction — quite literally, with the flip of a switch.
The treatment — deep brain stimulation — has long been used for movement disorders like Parkinson’s. Now, the first clinical trial of DBS for methamphetamine addiction is being conducted at Shanghai’s Ruijin Hospital, along with trials for opioid addicts. Yan is the study’s first patient; for fear of losing his job, he asked that only his surname be published.
Western attempts to push forward with human trials of deep brain stimulation for drug addiction have foundered, even as China has emerged as a hub for this kind of research.
But the vast suffering wrought by the U.S. opioid epidemic may be changing the risk-reward calculus for doctors and regulators. Now, the experimental surgery Yan underwent is coming to America. In February, the U.S. Food and Drug Administration greenlighted a clinical trial in West Virginia of DBS for opioid addicts.
Until now, complex ethical, social and scientific questions made it hard to push forward with such experiments in the United States, where the devices can cost $100,000 to implant. Scientists in Europe have struggled to recruit patients for their DBS addiction studies.
Globally, there are eight registered DBS clinical trials for drug addiction, according to a U.S. National Institutes of Health database. Six are in China.
China has a long, troubled history of using brain surgery to treat addiction. Doctors destroyed small clumps of tissue in the brains of heroin addicts, garnering huge profits and leaving behind a trail of patients with mood disorders, lost memories and altered sex drives.
This Monday, Oct. 29, 2018 photo shows a brain scan of a methamphetamine addict with the path of electrodes that doctors at Ruijin Hospital in Shanghai, China implanted to stimulate an area of the brain associated with addiction. AP
In 2004, China’s Ministry of Health ordered a halt to the practice at most hospitals. Nine years later, a military hospital in Xi’an reported that roughly half of 1,167 addicts who had their brains lesioned stayed off drugs for at least five years.
DBS builds on that history. The surgery involves implanting a device that acts as a kind of pacemaker for the brain, electrically stimulating targeted areas. Instead of irreversibly killing brain cells, the devices allow interventions that are — in theory — reversible. The technology has opened a fresh field of human experimentation globally.
“For many other psychiatric disorders, for example, anorexia schizophrenia, OCD, there’s no way to use the animal to be like a model,” said Dr. Sun Bomin, director of the functional neurosurgery center at Ruijin Hospital. “For these kinds of special psychiatric disorders we have to use human patients.”
Some believe such human experiments on drug addicts should not be allowed.
Critics argue that they are premature, and will not address the complex biological, social and psychological factors that drive addiction. Scientists don’t fully understand how DBS works and there is still debate about where electrodes should be placed to treat addiction. There is also skepticism in the global scientific community about the general quality and ethical rigor of clinical trials done in China.
Dr. Li Dianyou uses a tablet computer to adjust the settings of a deep brain stimulation device implanted in the brain of a methamphetamine addict named Yan, left, on Monday, Oct. 29, 2018, at Ruijin Hospital in Shanghai, China. AP
“It would be fantastic if there were something where we could flip a switch, but it’s probably fanciful at this stage,” said Adrian Carter, who heads the neuroscience and society group at Monash University in Melbourne. “There’s a lot of risks that go with promoting that idea.”
Meanwhile, the body count from addiction is rising. More than 500,000 Americans died of drug overdoses in the decade ending in 2017, adding urgency to the search for new, more effective treatments.
But research for DBS as an addiction treatment funded by the U.S. National Institutes of Health has focused on animals, not people. And at least two U.S. laboratories dropped clinical trials of DBS for treating alcoholism over concerns about study design and preliminary results that didn’t seem to justify the risks, investigators who led the studies told The Associated Press.
“The lack of scientific clarity, the important but strict regulatory regime, along with the high cost and risk of surgery make clinical trials of DBS for addiction in the U.S. difficult at the present time,” said Dr. Emad Eskandar, chairman of neurological surgery at Albert Einstein College of Medicine in New York.
China’s studies have offered mixed results. Sun and his colleagues have published one case study, describing a heroin addict who fatally overdosed after getting DBS. But a separate pilot study published in January by doctors at a military hospital in Xi’an showed that five of eight heroin addicts stayed off drugs for two years after DBS surgery.
Based on those results, the Chinese deep brain stimulation device manufacturer SceneRay Corp. is seeking Chinese regulatory approval of its DBS device for addiction, and funding a clinical trial targeting 60 heroin addicts. SceneRay chairman Ning Yihua said his application for a clinical trial in the U.S. was blocked by the U.S. Food and Drug Administration.
But in February, the FDA greenlighted a separate trial of DBS for four opioid addicts. The study lead, Dr. Ali Rezai, director of the West Virginia University Rockefeller Neuroscience Institute, hopes to launch the trial in June.
“People are dying,” Rezai said. “Their lives are devastated. It’s a brain issue. We need to explore all options.” Yan is among those whose lives have been ravaged. Years of drug use cost him his wife, his money and his self-respect, before landing him at Ruijin Hospital in search of a radical cure.
After surgery, Yan said the machine in his brain was magical. “It controls your happiness, anger, grief and joy,” he said. More than six months later, he says he is still off drugs and has put on 20 pounds. Sometimes, in his new life, he touches the hard cable in his neck that leads from the battery pack to the electrodes in his brain. And he wonders: What is the machine is doing inside his head?