European Medicines Agency recommends licensing Ebola vaccine
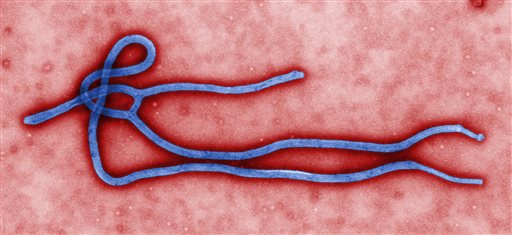
This undated file image made available by the Centers for Disease Control (CDC) shows the Ebola virus. AP
LONDON — The European Medicines Agency has recommended that the world’s first Ebola vaccine be approved, after it was administered to hundreds of thousands of people in Africa.
The agency on Friday described licensing the vaccine as “an important step toward relieving the burden of this deadly disease.”
The Ebola vaccine was originally developed in Canada and is now marketed by Merck as Ervebo. More than 270,000 people in Africa have received it as officials try to stop Congo’s ongoing outbreak.
A second vaccine made by Johnson & Johnson, which is not yet licensed, will soon be used in parts of Congo where Ebola is not actively spreading.
Also Friday, the World Health Organization is convening a meeting to consider whether the epidemic in Congo should still be designated a global emergency. IB/JB
RELATED STORIES:
Second Ebola vaccine to be used in DR Congo next month
Ebola survivors at higher risk of dying, even after recovery
Filipinos in Congo advised to remain cautious amid ebola outbreak